Why Is Fluorine the Most Reactive Element in Group 7
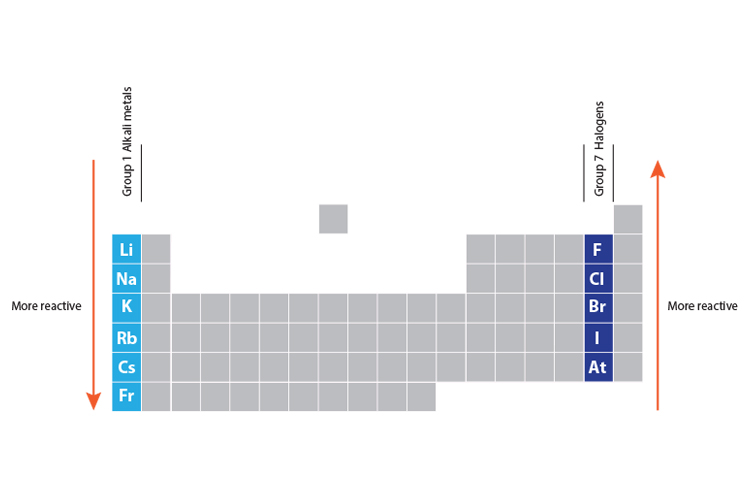
Fluorine is the most reactive element of all in Group 7. The elements in group 7 are called the halogens.

Group 7 Halogens Gcse The Science Hive
Fluorine is the most reactive element in Group 7 and is even more reactive thanchlorine.
. This weaker attraction in the larger atoms makes it harder to gain electron. We say that fluorine is electronegative. Why is Cl more reactive than Br.
Fluorine is the most reactive element of all in Group 7. Fluorine is the most reactive element in Group 7 and is even more reactive than chlorine. The atoms become bigger and the outer shell of electrons is further away.
Fluorine has the least shielding therefore it can gain an electron to the outer shell more easily. Click to see full answer. Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron is greater and so.
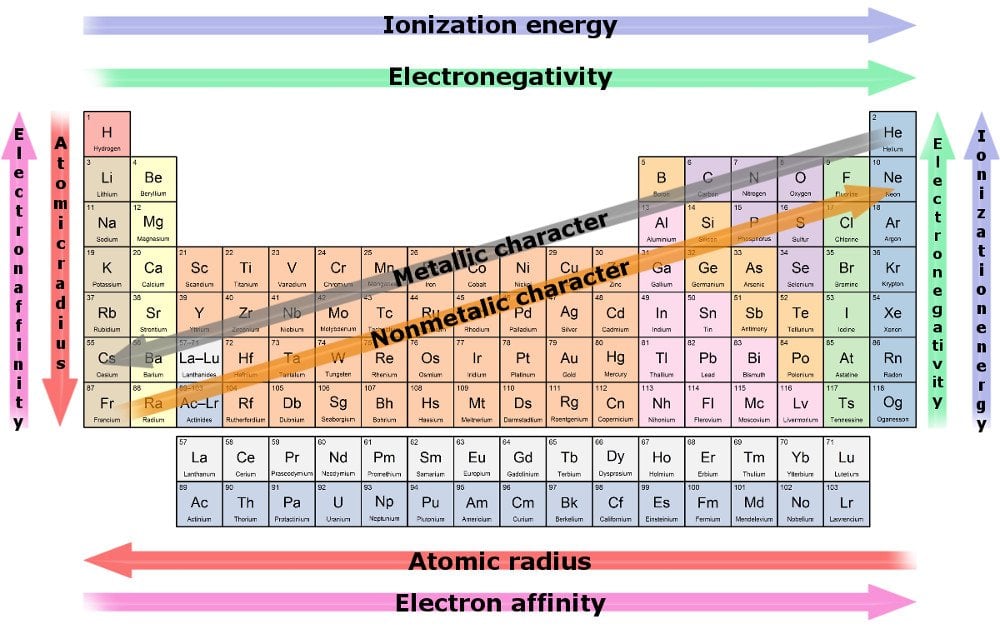
Since electronegativity decreases going to the left of the periodic table oxygen is the second most reactive element on the list. Which is the most reactive element in halogen group. The electrons in the outer shell move further away from the nucleus as we go down the group and the attraction force between the electrons and the nucleus become weaker and weaker.
Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron is greater and so. Fluorine is the most reactive element of all in Group 7. Does fluoride cause iodine deficiency.
Mezitis agreed that while fluoridation of the water supply is important for dental health studies have also shown that iodine deficiency that may be caused by extra ingestion of. Fluorine is the most reactive element in Group 7 and is even more reactive than chlorine. 4 rows Fluorine is the most reactive element of all in Group 7.
Explain in terms of electronic structure why fluorine is the most reactive element in Group 7. Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron. Fluorine has the smallest distance between the outer shell and the electrons.
You can see the trend in reactivity if you react the halogens with iron wool. It is the lightest halogen. The non-metal elements in Group 7 known as the halogens get less reactive as you go down the group.
Halogens are reactive because the outer shells that orbit the nucleus lack electrons. The fluoride the negative ion of the element fluorine easily displaces iodine in the body because it is much lighter and therefore more reactive. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic table.
All halogens form Group 1 salts with similar properties. Fluorine is the most reactive element in Group 7 and is even more reactive than chlorine. Why does Bond enthalpy decrease down Group 7.
The reactivity of Group 7 elements decreases down the group. Answer Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron is greater and so it can gain an extra electron more easily. An element with 7 valence electrons is more reactive because allelements want to have 8 valence electrons.
Within this group helium is the least reactive element forming no stable compounds. This is because the elements need to gain an electron to form a more stable compound. In these compounds halogens are present as halide anions with charge of -1 eg.
Are the group 17 elements highly. You can see the trend in reactivity if you react the halogens with iron wool. Which Halogen is most reactive.
As the period no. Metalloids are generally less reactive than metals and nonmetals. Visit the BBC Bitesize website to read more about this and then explain below why fluorine is more reactive than chlorine.
The reaction is faster than that of iodine but slower than that of chlorine. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic table. Why is fluorine more reactive.
Fluorine is a pale yellow diatomic highly corrosive flammable gas with a pungent odor. What is the trend in reactivity in Group 7. The halogens show trends in their physical and chemical.
Fluorine is the most reactive element of all in Group 7. Although the bromine nucleus is more positively charged than the chlorine nucleus the increase in the radius and the extra shielding in the bromine atom outweigh this factor which means that an electron is more easily attracted into the outer shell of a chlorine atom than that of a bromine. As a general rule fluorine is the most reactive halogen and astatine is the least reactive.
As you go down the group to chlorine bromine etc. Fluorine Fluorine is the most reactive element of all in Group 7. Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron is greater and so it can gain an extra electron more easily.
What is the most reactive element in Group 7. Fluorine is the most reactive because it has the strongest attraction for oxygen. The one parts it would not vigorously react with are oxygen helium neon and argon.
Fluorines outer shell is closer to the nucleus and has fewer filled shells between it and the nucleus so the attraction for a new electron is greater and so. With halogens the higher an element is in the column the more reactive it is. Fluorine is the most reactive of the halogens because it is at the top of the halogen group which is the second to right group on the periodic table.
Fluorine is the most reactive element in Group 7 and is even more reactive than chlorine. Group 7 elements have 7 outer electrons and need to gain one electron. It is without doubt one of the few parts that can kind compounds with noble gases xenon krypton and radon.
Fluorine is the most reactive and the most electronegative of all the elements. If an element only has7 it can react to get to 8. You can see the trend in.
What do the elements in Group 7 have in common. Fluorine is essentially the most reactive and most electronegative of all of the chemical parts.
Which Of The Above Elements Are Most Reactive And Why Quora
Which Is The Most Reactive Element In The Periodic Table Science Abc
Which Is The Most Reactive Element In The Periodic Table Science Abc
Periodicity Of Group 7 Reactions Activity

Group 7 Halogens Fluorine Chlorine Bromine Iodine Physical Properties Balanced Equations Chemical Reactions Balanced Gcse Chemistry Revision Notes Ks4 Science Igcse O Level

Group 7 Elements By Yazan Dabbagh Introduction Group 7 Elements Are Commonly Referred To As Halogens There Are Five Elements And They Include Fluorine Ppt Download

Zinc Element Symbol Properties Facts Compounds Uses In 2021 Chemistry Basics Zinc Element Facts

As You Move Down Group 1 And 7 Elements Get More Reactive

David Chalk Teacherchalky1 Twitter Gcse Science Science Daily Science Revision

Reactivity Of Group 17 Elements Periodic Table Youtube

Fluor Wikipedia Element Symbols Element Chemistry Science Chemistry

Halogen Elements Examples Properties Uses Facts Britannica

Group 7 Gcse Chemistry Combined Science Aqa Revision Study Rocket

Which Of The Above Elements Are Most Reactive And Why Quora

Why Is Fluorine The Most Reactive Element In Group 7 Brainly In
What Is The Most Reactive Element In The Periodic Table That Reacts To All Substances Quora


Comments
Post a Comment